
Tablets and capsules are mainstay product forms, so what are the spending and innovation trends for solid-dosage manufacturing equipment and machinery?

Tablets and capsules are mainstay product forms, so what are the spending and innovation trends for solid-dosage manufacturing equipment and machinery?

In Part I of this article, which appeard in the March 2010 issue, the authors describe their approach for constructing form spaces for carbamazepine, cimetidine, and phenylbutazone by initial solvent screening to evaluate the feasibility of spherical crystallization. Part II of this article discusses their findings.

Vibrational spectroscopy, which encompasses near-infrared, mid-infrared and Raman spectroscopy, is an important analytical tool in the pharma industry.
In Part I of this article, the authors describe the materials and methods used in developing a screening strategy to accelerate the preparation and characterization of spherical agglomerates by spherical crystallization.

Capsules that can release their contents at a selected temperature have been developed by researchers in France, and could lead to the development of therapeutic agents that are applied to the skin and triggered locally by rubbing.

Also, Merck & Co. extends collaboration with Idera Pharmaceuticals; Pfizer establishes R&D center in China; more...
Leading experts share insight on polymorphism and crystallization.

Manual sample preparation methods for solid dose pharmaceuticals have a number of inherent disadvantages, primarily because they are time consuming and unpredictable.

Following its $68-billion acquisition of Wyeth, Pfizer has unveiled detailed plans for its global R&D network, which includes consolidation of its R&D facilities.

Following the its $68-billion acquisition of Wyeth (Madison, NJ), Pfizer (New York) Pfizer detailed plans for its global research and development (R&D) network, which includes consolidation of its R&D facilities.

A smart nanocage that can be filled with a medicinal substance and then activated using light has been developed by US researchers.

A new drug delivery method developed by scientists could enable prescription drugs to be buried inside the body where drug release could be prompted by a biological trigger, such as a drop in blood sugar levels, or activated manually with a pulse of light.
The authors investigated the effects of formulation and processing parameters on floating matrix-controlled drug-delivery systems.

The author discusses control strategies via near infrared instrumentation for continuous mixing, granulation, drying, and extrusion with a more focused detail on mixing.

Semisolid Formulation Development: The CRO Approach - SP Formulations Whitepaper

The authors describe the various available technologies used in orally disintegrating tablets.

NIR Prediction of Solid Dosage Form Dissolution Profiles - Foss Whitepaper

Extending Calibrations for Near-Infrared Assay of Tablets Using Synthetic Modeling and Variance from Placebos - Foss Whitepaper
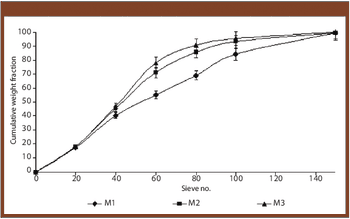
The authors prepared granules containing cinnarizine using polyethylene glycol 6000 as a melting binder and lactose monohydrate as hydrophilic filler. The effects of binder concentration and size were studied.

Bulk Inspection of Tablets-Symetix Applicaton Note
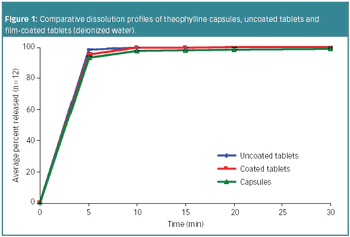
Based on formulation simplicity and blinding capability, hard gelatin capsules are preferrable compared with other oral solid dosage forms, including tablets, in the early clinical phases of drug development.
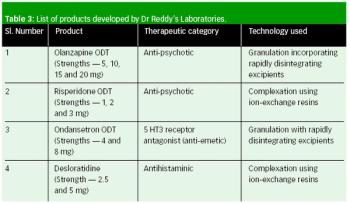
Orally disintegrating tablets offer numerous advantages compared with traditional tablets and capsules, and can be an effective solution for developing line extensions of currently marketed therapies.

Bulk Inspection of Tablets-Symetix Application Note

The authors examine common superdisintegrants (i.e., crospovidone Type A, crospovidone Type B, croscarmellose sodium, and sodium starch glycolate) with a set of poorly soluble drug actives and evaluate in vitro drug dissolution.
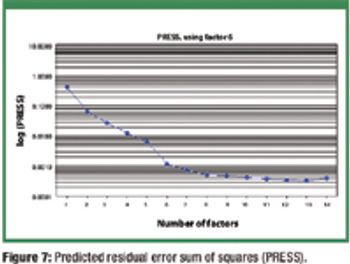
The authors extend the range of a near-infrared calibration model for tablet assay using production 'seed' spectra and synthetic spectra generated from placebos and 'pure' active pharmaceutical ingredient spectra.