
IPEC's new stability testing guide takes into account the full supply chain's storage conditions.

IPEC's new stability testing guide takes into account the full supply chain's storage conditions.

Parteck ODT is a newly introduced ready-to-use excipient for fast melt tablets.
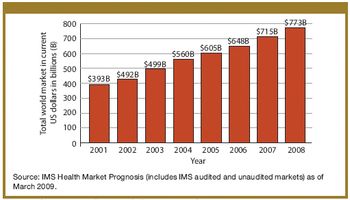
Biologics enhance their positions amidst slowing growth in the global and US markets.

The author of a book about biopharmaceutical production includes irrelevant information.

The US Pharmacopeia is revising its monographs for four pharmaceutical excipients: propylene glycol, sorbitol solution, sorbitol sorbitan solution, and noncrystalllizing sorbitol solution.

Formulators and manufacturers have many options for modifying release profiles in multiple-API products.
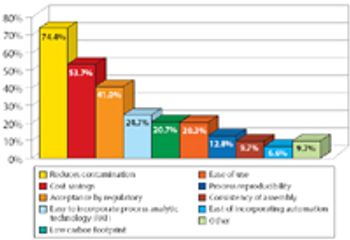
A Pharmaceutical Technology report looks at trends in biopharmaceutical manufacturing. This article contains bonus online-exclusive material.
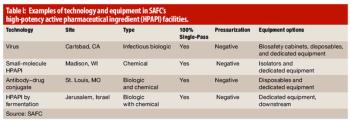
High-potency manufacturing of active pharmaceutical ingredients is a growing and specialized capability.

A look at the true cost-drivers of cell-culture production.

The emergence of influenza A (H1N1) and the efforts to provide vaccines to the vulnerable are timely examples of biopharmaceuticals' continuing importance.

The European Commission's (EC) Directorate-General for Enterprise and Industry (EC-DG Enterprise) announced last week that it will not continue preparing a commission directive on good manufacturing practices (GMPs) for certain excipients.

CROs and CMOs expand to gain a piece of the market for clinical trial materials.

Today, approximately 1.5 million counterfeit medicine packs enter the legal supply chain each year - in other words, one pack in every 20000 is counterfeit.

Strong growth in biopharmaceuticals bodes well for contract manufacturing, but the perils and the promises of pipelines remain.
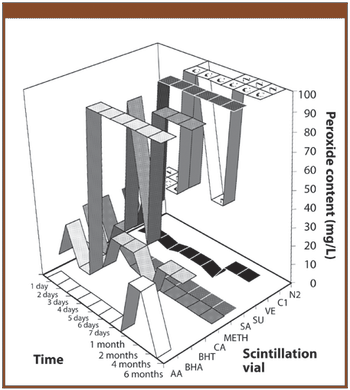
The authors investigate whether the addition of an antioxidant could be used to stabilize the solvent ethyl lactate by preventing the formation of peroxides

The GDP committee of IPEC–Europe is trying to seal one more broken link in the supply chain. This article contains bonus online-exclusive material.

The organizations' presidents discuss market exclusivity, approval processes, and pending legislation.

2009 Post-Interphex Showcase: Chemicals
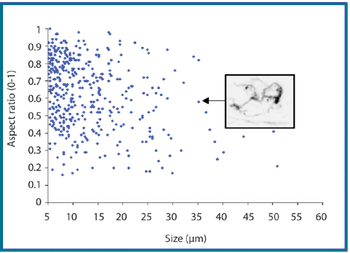
The authors describe a novel analytical approach that uses the shape-analysis capabilities of MFI to detect and enumerate silicone oil microdroplets in protein formulations that also contain aggregates of similar size and in a similar concentration.

A review of recent product innovations, policy developments, and growth prospects in the excipients market.

The spotlight on the biopharmaceutical industry is intensifying, as recently evidenced by Pfizer's (New York) ongoing acquisition of Wyeth (Madison, NJ), which was initiated partly to reduce the former's dependence on small-molecule drugs.

Ultra high performance liquid chromatography is advantageous in a contract laboratory because it is faster, more sensitive, and relies on smaller volumes of organic solvents than HPLC.

Contract manufacturers deploy a business model using operations in the US and Western Europe with facilities in Asia.

Without any GMP guidelines for excipients in Europe, change can't come soon enough for some industry groups.
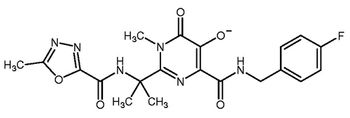
The pharmaceutical majors forward projects in biocatalysis, solvent replacement, and other approaches in green chemistry.