
Optimizing the solid form of a drug reaps scientific and technical awards.

Optimizing the solid form of a drug reaps scientific and technical awards.

A well-balanced guide to industrial bioseparations provides valuable information.
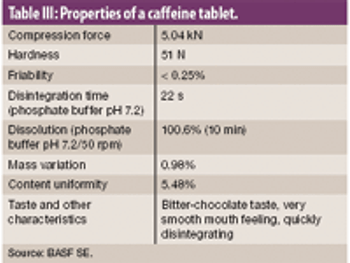
The authors examine the effectiveness of an excipient comprised of mannitol, polyvinyl acetate, and crospovidone using model actives loperamide hydrogen chloride and caffeine.
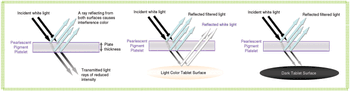
Sophisticated excipient development, especially for coatings, is staying on top of new challenges and meeting expanding industry needs.

IPEC Chairman Dave Schoneker discusses current efforts toward facilitating regulatory reviews of new excipients.

Molecules called "chaperones" facilitate correct protein folding.

The European Fine Chemicals Group (EFCG) and the International Pharmaceutical Excipents Council of Europe (IPEC Europe) announced the formation of a European Pharmaceutical Excipients Certification Project to develop advocacy and stakeholder management in Europe and to give advice to two European working teams as part of an effort to develop a certification program for manufacturers and distributors of pharmaceutical excipients.

Emerging pharmaceutical companies represent an important client base for CROs and CMOs. Lessons learned for successful customer–supplier relations.
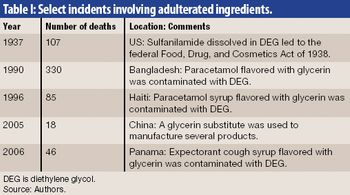
Securing the integrity of the excipient supply chain is a crucial task in ensuring the overall pharmaceutical supply chain. The authors outline excipient-control strategies and practices for the manufacture, distribution, and receipt of excipients.

The potent nature of HPAPIs means there must be careful evaluation of the compound for its level of toxicity when considering manufacture.
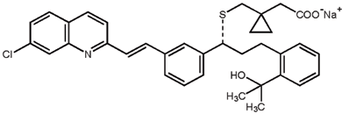
Carbon�–hydrogen functionalization, ketone α-alkylation, and biocatalysis are some recent advances in asymmetric synthesis.
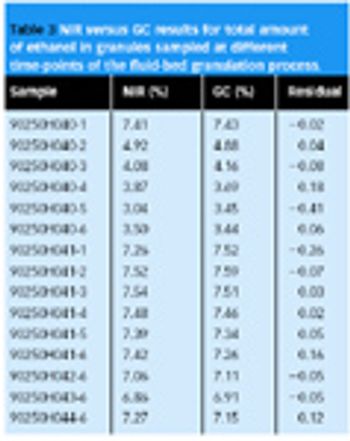
Near-infrared spectroscopy (NIR) is suitable for the analysis of pharmaceutical samples in various solid forms, and can be used for determining chemical properties (e.g., content of drug, water), as well as physical properties (e.g., particle size, tablet hardness).

The authors describe the critical aspects of an ideal fermentation services provider.

CMOs expand capacity and capabilities in high-potency manufacturing to meet strong demand for cytotoxic and other potent drugs.

Pharmaceutical companies developing new drug candidates for Hepatitis C virus infection now can test their compounds with a novel culture system that mimics the biology of HCV infection in humans.
Drugmakers seeking to block the activity of a protein may have a new strategy at their disposal.

A roundtable with John Doney, Jiao Yang, Hans Baer, and Elena Draganoiu.

Clear labels for substances that can be used as excipients, APIs, or both are critical to end-product use.
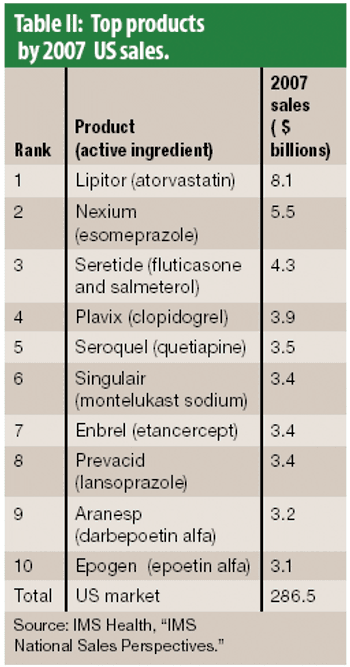
The year 2007 was slow for approvals for new molecular entities and overall pharmaceutical industry growth. Big Pharma seeks relief in a growing biologics portfolio.

Antibody drug conjugates offer a niche opportunity in drug development and contract manufacturing.
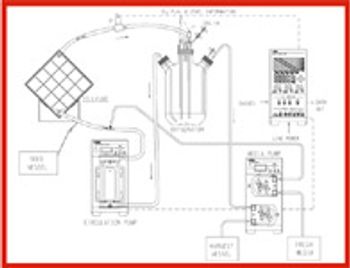
Creating a kinder, gentler manufacturing process that doesn't kill the product is the goal of process developers doing large-scale cell culture for cell therapy.

Nanoparticle-based systems present many advantages for the delivery of current and emerging biological drugs.

Polyplus-transfection, a company that researches, develops, and commercializes drug-delivery solutions for biomolecules, created a new technology designed to enhance in vivo delivery of small interfering RNAs (siRNAs) when they are associated with a cationic polymer.

The less complex nature of excipient manufacturers, as compared with API manufactures, carries many benefits.
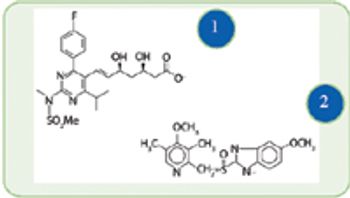
Chemocatalytic and biocatalytic routes show promise for more efficient syntheses of select active ingredients.