
Optimizing the solid form of a drug reaps scientific and technical awards.

Optimizing the solid form of a drug reaps scientific and technical awards.

Despite challenges, contract manufacturers in Europe are enjoying considerable success.
The authors examine the effectiveness of an excipient comprised of mannitol, polyvinyl acetate, and crospovidone using model actives loperamide hydrogen chloride and caffeine.
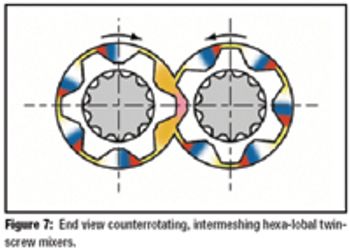
The author describes the benefits, processes, and practicality of using hot-melt extrusion to mix active pharmaceutical ingredients with pharmaceutical-grade polymers.
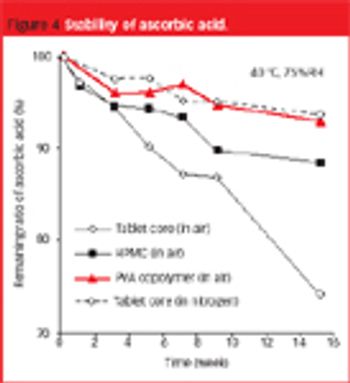
The use of PVA copolymer-based film can solve the problems associated with lack of film adhesion... to tablets containing large amounts of waxy excipient or a lubricant.

USP announced an interim revision to its monograph for levothyroxine sodium tablets, which will become official in USP 32-NF 27.
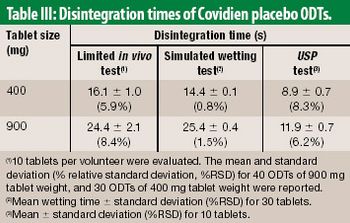
The authors propose an alternative to the USP disintegration test method. The method embraces physiological conditions of the oral cavity, as a screening tool for developing ODT products.

3M Drug Delivery Systems has successfully designed a proof of concept device using a solid microstructured transdermal system for the systemic delivery of high-potency pharmaceuticals. The technology was showcased at a poster session at the annual meeting of the Controlled Release Society held this week in New York City.
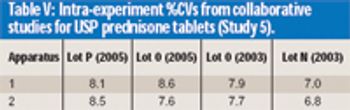
The authors demonstrate that anecdotal reports of prednisone tablet variability are inaccurate.
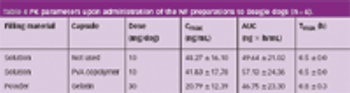
When drugs are encapsulated, electrification (the electrostatic charge of the capsule) may sometimes cause problems, such as capsule adhesion during transportation or dispersion of the capsule content in the filling process.

Recent advances in SEM, particularly the incorporation of automation and software, have made simpler, lower-end SEM instruments easy to operate and have improved the capabilities of larger, sophisticated instruments.

Traditional tablet presses do not measure tablets' tensile strength, yet this characteristic strongly influences tablet quality. The author describes a compression technique that accounts for tensile strength and produces tablets with consistent weight and disintegration time.
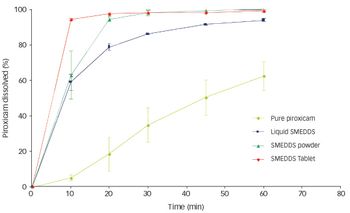
Spraying techniques can be used to produce powder form formulations. The concept works by the adsorption/absorption of a liquid SELF onto a neutral carrier…

Chemical imaging of solid dosage forms has become a powerful analytical tool for the development of solid dosage forms.

To ensure an effective treatment, a patient often must take equal doses of an active pharmaceutical ingredient (API) at regular intervals.

We are currently experiencing a problem with one of our tablet lines. While the tablets appear white immediately after manufacture, after a time many of the tablets begin to take on a yellowish appearance. Could this be an issue that surface analysis could help resolve?

Natural gums and mucilage are biocompatible, cheap, readily available, and represent a potential source of excipients. The authors examine the functionality of mucilage extracted from the leaves of Hibiscus rosa-sinensis Linn as an excipient in a sustained-release tablet formulation.

Individualized dosing for specific patient needs has been the goal of medical and pharmacotherapy specialists since they first envisioned pharmacogenetic evaluation. With the measurement of individual levels of metabolism, the optimum dose can be calculated for each individual patient.
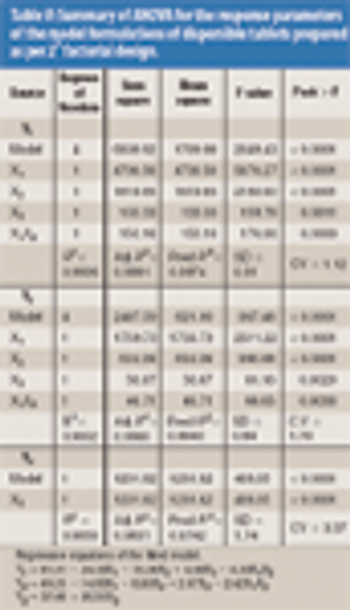
The authors analyzed the effects of complexation as well as the levels of ammonium bicarbonate and crospovidone on tablet wetting time (WT), disintegration time (DT), and percent dissolution efficiency at 60 min (%DE60).
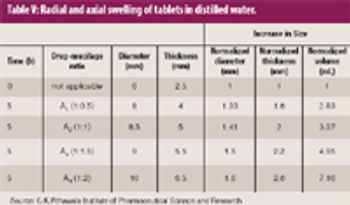
Natural gums and mucilage have been widely explored as pharmaceutical excipients. The goal of this study was to extract mucilage from the leaves of Aloe barbadensis Miller and to study its functionality as an excipient in pharmaceutical sustained-release tablet formulations.

The crystalline structure of pharmaceutical solids can sometimes be altered during processing. X-ray powder diffraction and near infrared spectroscopy can be used to determine the amorphous and crystalline content of a model substance. The two techniques' precision, accuracy, detection limit and the speed of analysis are compared.

Ranbaxy received full market approval from the US Food and Drug Admnistration for its anti-infective agent ?Clarithromycin? oral suspension.
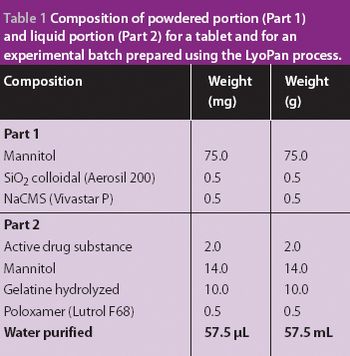
A new economical method for producing fast-melting lamina-like dosage forms.
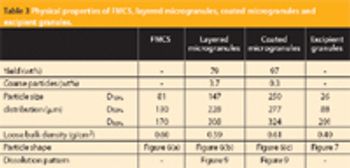
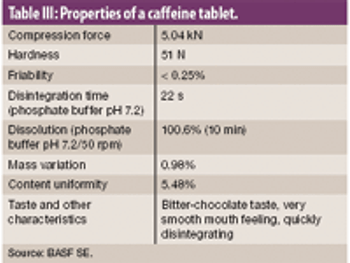
This article describes how rapidly disintegrating tablets containing a large quantity of an intensely bitter drug were successfully developed with a suitable level of masking, tablet hardness, disintegration property, dissolution profile and mouth feel.